Summary: Although opioids are frequently used to manage persistent cancer pain, the new study finds that there is insufficient data to show that they are as effective. There appear to be very few RCTs confirming their efficacy in cancer pain. Most studies on the topic are either small or outdated.
Opioids have long been the cornerstone of cancer pain management. However, this new study reveals a surprising lack of placebo-controlled trials supporting their efficacy. This gap in evidence is particularly notable for widely used opioid analgesics such as morphine, methadone, buprenorphine, fentanyl, hydromorphone, oxycodone, and tramadol. The largest placebo-controlled trial in this area involved tapentadol, demonstrating a higher response rate than placebo, though the treatment effect size was unclear. The existing studies for codeine suggest some benefit for mild-to-severe cancer pain, but these studies were generally small and not reflective of typical clinical use.
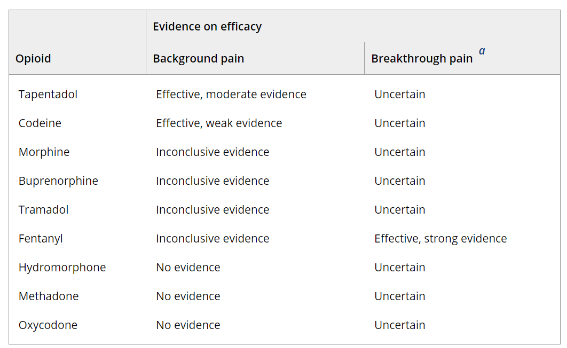
A summary of evidence (Table 1 Below) indicates varying levels of efficacy for different opioids: moderate evidence supports tapentadol for background pain, while fentanyl shows strong evidence for breakthrough pain. However, the evidence is inconclusive or lacking for many other commonly used opioids. This lack of robust data complicates the formulation of treatment guidelines, especially considering the diverse types and stages of cancer pain and the need for different approaches for chronic cancer pain versus end-of-life care.
Table 1 Summary of evidence on the efficacy of opioid analgesics compared with placebo.
Morphine is frequently recommended as the first-line treatment for moderate-to-severe cancer pain due to its cost-effectiveness and perceived benefits. However, its administration can be challenging in patients with hepatic or renal impairment, given its metabolism and excretion pathways. Efficacy trials outside of end-of-life care show conflicting results for morphine when compared with placebo, and more studies are needed to clarify its role and effectiveness.
This latest study also emphasizes the potential of non-opioid alternatives and adjunctive therapies for cancer pain. NSAIDs, such as aspirin, piroxicam, ketorolac, and diclofenac, have shown efficacy comparable to opioids for moderate-to-severe cancer pain. This suggests that these non-opioid options could be valuable, especially for patients seeking to avoid opioids or reduce opioid doses due to the risk of tolerance and dependency. However, most of these studies are outdated and involve small participant numbers, necessitating further research to confirm these findings.
Opioid prescribing in cancer pain management needs careful consideration of potential adverse outcomes, such as opioid use disorder, hyperalgesia, tolerance, and dependency.
Further, the potential immunosuppressive effects of opioids, particularly morphine, have been a growing concern, though current studies are limited by design issues such as confounding bias. The association between opioid use and risks like infection or tumor progression remains uncertain, prompting calls for well-designed and more extensive clinical studies.
There is also a need for a better understanding and subclassification of cancer pain to tailor opioid therapy more effectively. Nonpharmacologic treatments, either alone or in combination with analgesics, should be explored more thoroughly as potential pain management strategies. Additionally, more research is needed on therapies to mitigate opioid-induced toxicities, such as antiemetics and laxatives, to enhance patient care and treatment outcomes.
In conclusion
The new study shows that there is a significant gap in evidence for the efficacy of commonly used opioid analgesics in cancer pain management. There is a critical need for more rigorous trials with clearly defined populations to guide better-informed decision-making.
Source:
Abdel Shaheed, C., Hayes, C., Maher, C. G., Ballantyne, J. C., Underwood, M., McLachlan, A. J., Martin, J. H., Narayan, S. W., & Sidhom, M. A. (2024). Opioid analgesics for nociceptive cancer pain: A comprehensive review. CA: A Cancer Journal for Clinicians, 74(3), 286–313. https://doi.org/10.3322/caac.21823




